Overview of eCTDValidator
eCTDValidator by REGAPPS, is a regulatory validation application, designed to support validation of both eCTD and non-eCTD electronic submission formats.
eCTDValidator ensures technical compliance by validating submissions against the latest global health authority rules and guidelines—including those from the US-FDA, EU-EMA, and other international agencies.
Seamlessly integrated within ViewerPlus, eCTDValidator enables real-time validation directly in the viewing environment. With continuous updates aligned to evolving regulatory requirements, it helps teams stay ahead of compliance changes and avoid costly submission errors.
Note: Download our fully functional software products for free and explore application capabilities. Your feedback drives our innovation — helping us tailor solutions that truly meet your needs.
The following images offer a glimpse into the eCTDValidator powered by ViewerPlus interface and its key features.
| 1. Validator Workspace screen |
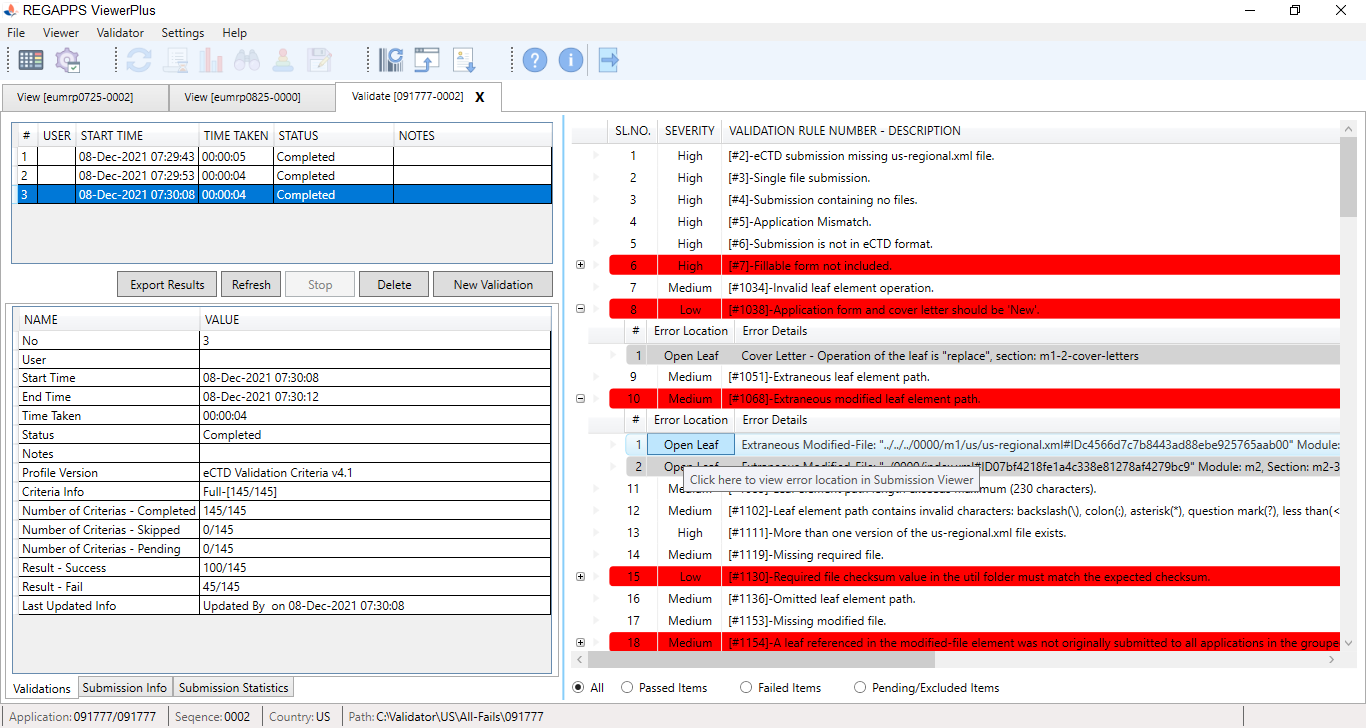
|
|---|---|
| 2. Tool to update MD5 for eCTD Submission |
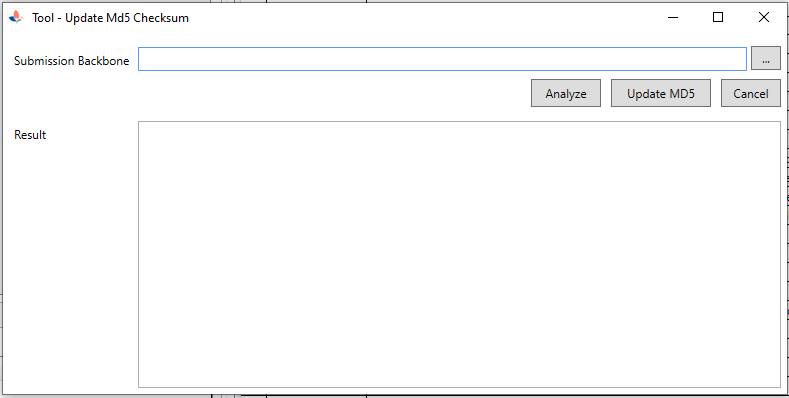
|
| 3. Tool to validate XML against DTD or Schema |
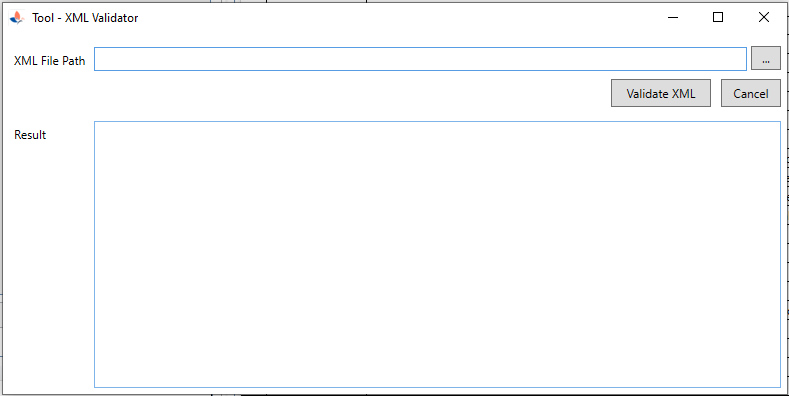
|
| 4. Validation Report |
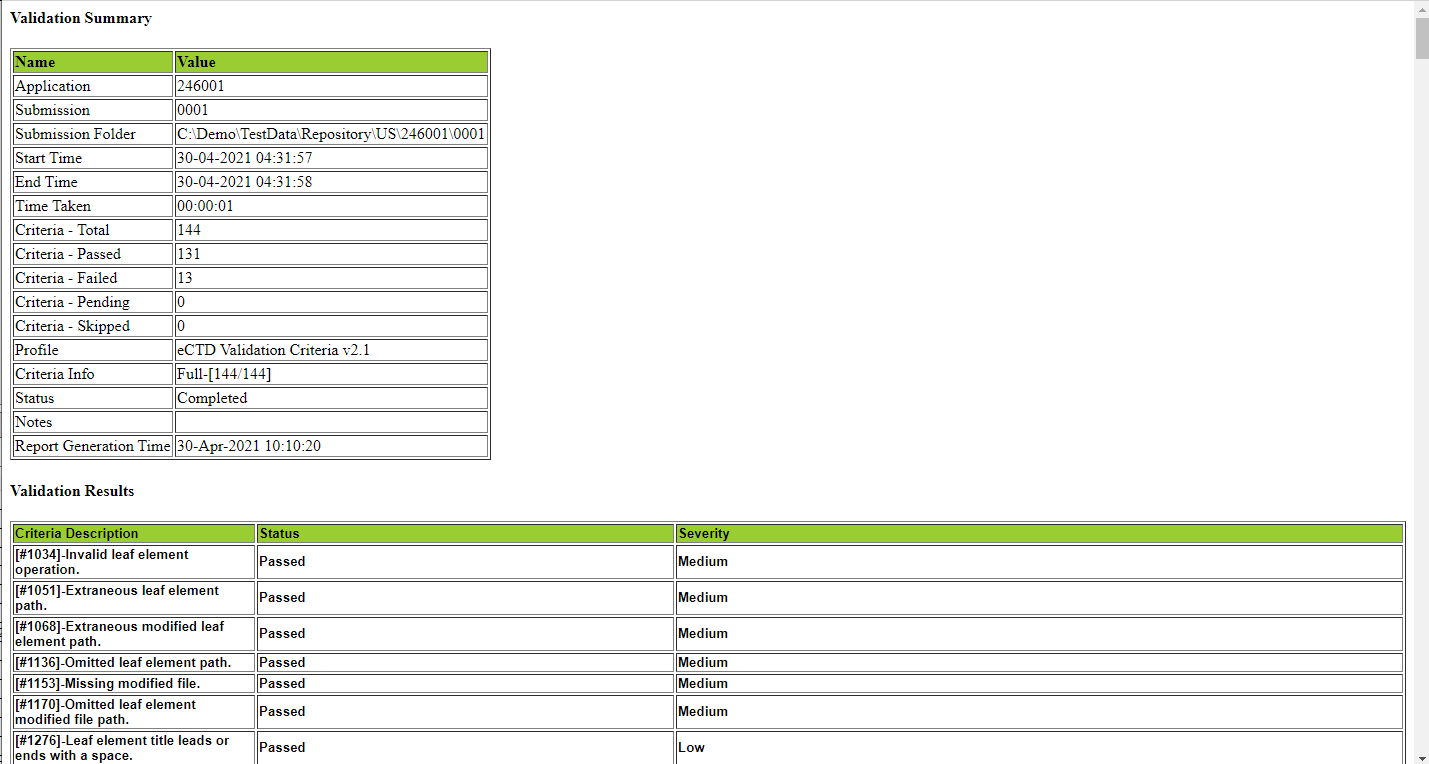
|